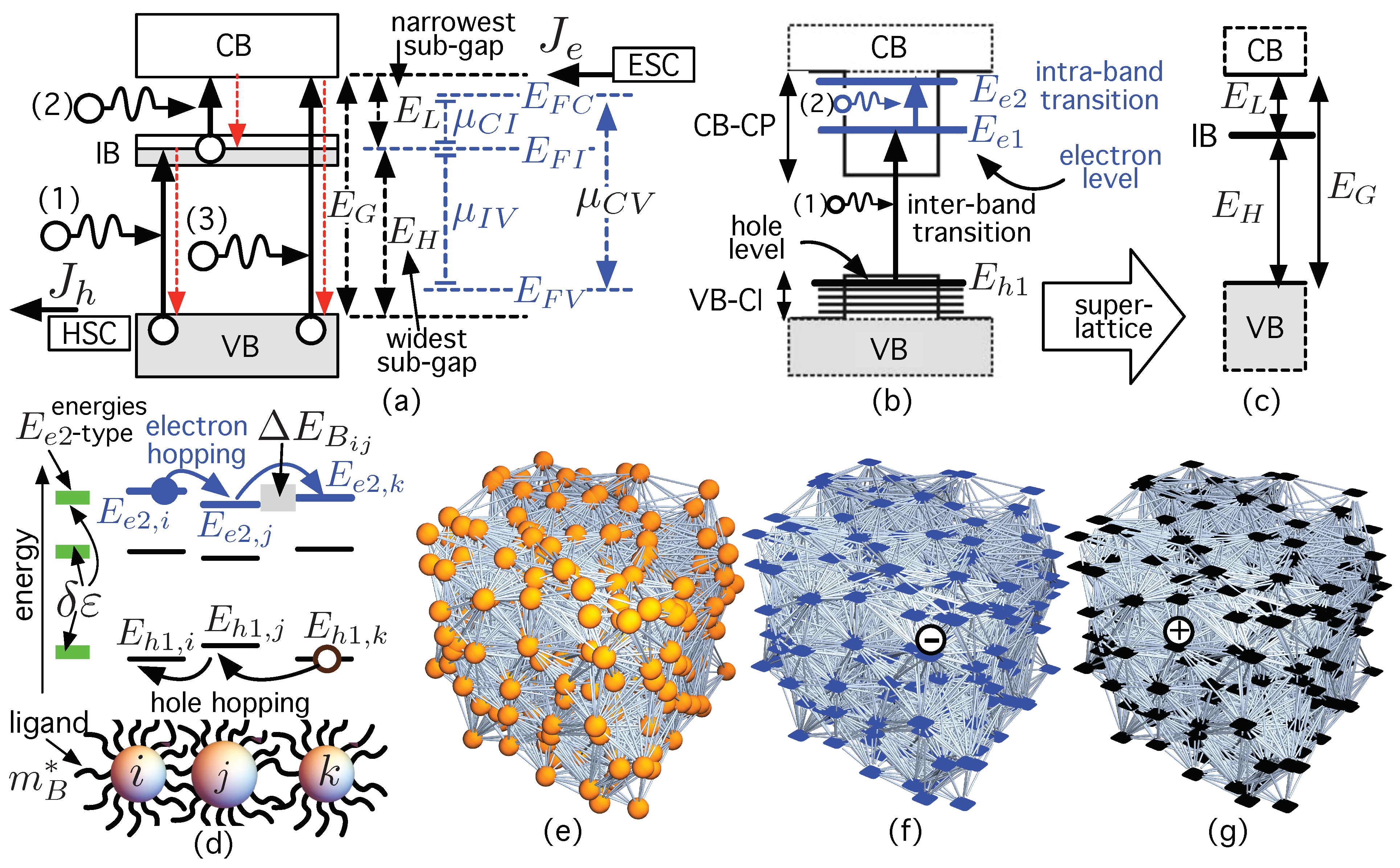
IJMS | Free Full-Text | Carrier Transport in Colloidal Quantum Dot Intermediate Band Solar Cell Materials Using Network Science
Given: The mass of electron is 9.11 x 10^-31 kg, Planck constant is 6.626 x 10^-34 J s, - Sarthaks eConnect | Largest Online Education Community

The mass of electron is 9.11 × 10^-31 kg.Planck's constant is 6.626 × 10^-34 Js then the uncertainty involved in the measurement of velocity within a distance of 0.1 A is :

Calculate the de - Broglie wavelength of an electron moving with one fifth of the speed of light. Neglect relativistic effects. ( h = 6.63 × 10^-34 J.s., c = 3 × 10^8 m/s , mass of electron = 9 × 10^-31 kg )

Physics of electron emission and injection in two‐dimensional materials: Theory and simulation - Ang - 2021 - InfoMat - Wiley Online Library

Intramolecular Electron-Transfer Rates in Mixed-Valence Triarylamines: Measurement by Variable-Temperature ESR Spectroscopy and Comparison with Optical Data | Journal of the American Chemical Society

Uncertainty in the position of an electron (mass = 9.1 × 10^-31kg) moving with a velocity 300ms^-1 accurate upon 0.001
![Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ] Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]](https://dwes9vv9u0550.cloudfront.net/images/8714373/032957da-34c2-47ec-a991-027613566c64.jpg)
Calculate the energy of the light having wavelength 45 nm : [Planck's constant h = 6.63 × 10^-34 Js ; speed of light c = 3 × 10^8 ms^-1 ]